Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states.

The reaction is a reaction between hydrogen gas and brown vapor of iodine monochloride. The reaction produces iodine and hydrogen chloride gases.
The proposed mechanism
We can propose a mechanism that involves 2 elementary steps for the reaction.
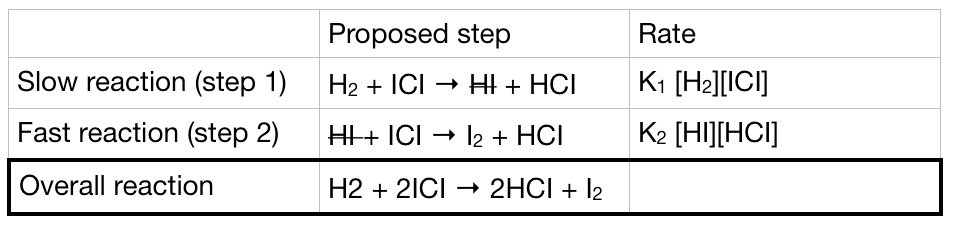
HI is an intermediate in the reaction and it does not appear in the final equation. The proposed mechanism supports the stoichiometry of the overall reaction so, it’s most likely that the reaction takes place in this mechanism.
Diagram
The reaction coordinate diagram is represented below:
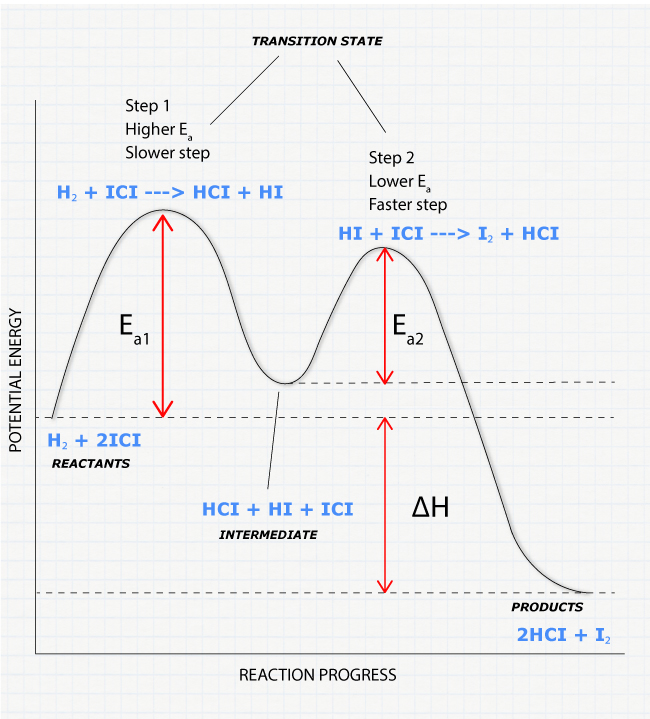
Things to note
- ΔH represents the difference between enthalpy of reactants and products.
- Ea1 and Ea2 represent the activation energy for step 1 and step 2 in the reaction.
- The step with the highest activation energy is the slowest step reaction.
- The step with the lowest activation energy is the fastest step in the reaction.
- The reaction cannot proceed faster than the rate of the slowest elementary step.

