Mastering VSEPR Shapes: Key Concepts for AP Chem
In the month of October, many teachers often find themselves tackling Unit 2.7 of their course materials in AP Chemistry. This unit, which focuses on ‘VSEPR‘ (Valence Shell Electron Pair Repulsion) theory and ‘Bond Hybridization’ for AP Chem. Educators typically use this time to introduce their students to the intricacies of molecular geometry, bond angles, and hybridization concepts, hitting the ground running as they lay the foundation for more advanced chemistry principles. For example, VSEPR theory is essential for understanding the molecular shapes and polarities that influence intermolecular forces (IMF).
In this article, we aim to provide a teacher-friendly guide to navigating “VSEPR for AP chem.” We’ll delve into Chapter Topic 2.7 VSEPR and Bond Hybridization and discuss the framework of the College Board’s Course and Exam Description (CED).
Problems teachers face
Teaching the concept of VSEPR shapes in chemistry can be both fascinating and challenging. Many teachers encounter difficulties when trying to convey the abstract nature of molecular shapes to their students. After all, molecules are not visible to the naked eyes. However, understanding these shapes is fundamental to comprehending chemical behavior and molecular interactions. In this chapter, we aim to assist educators in tackling this crucial topic effectively.
Connecting Molecular Shapes to the Real World: A Teaching Approach

To make VSEPR shapes more accessible and engaging for students, it’s crucial to bridge the gap between abstract theory and practical relevance. By helping students relate molecular shapes to their everyday experiences, we can introduce this fundamental concept with meaning and context. For instance, teachers can initiate the lesson by delving into how molecular shapes impact the properties of substances encountered in daily life, like water’s remarkable characteristics. Water’s ability to sustain life in freezing temperatures, explained by ice floating on water, its capacity to dissolve numerous substances, enabling various chemical reactions, can serve as a tangible example. Additionally, the importance of molecular shapes in drug design can be explored. Teachers can showcase different drugs and discuss their unique shapes, illustrating how molecular geometry influences their effectiveness. For example, many drugs work by inhibiting enzymes in the body. These drugs are designed with shapes that fit the enzyme’s active site, much like a key fits into a lock. These examples represent just the tip of the iceberg; myriad real-world applications underscore the significance of comprehending molecular shapes. By forging these connections, we can provide students with a deeper appreciation of the importance molecular shapes play in the world around them. After fostering meaningful discussions, teachers can then guide students in predicting the shapes of molecules, solidifying their grasp of this essential concept.
VSEPR for AP chem
VSEPR for AP Chemistry is a very important topic. The AP Chemistry standards demand that students develop a profound understanding of chemical phenomena and the capability to construct representations and models for various chemical concepts. In particular, students are expected to learn:
| Skills | Description |
|---|---|
| Interpret Graphical Representations | Students learn to interpret graphical representations of potential energy changes as atoms approach each other. They use these representations to explain optimal bond length and understand factors determining bond formation. |
| Construct Representations and Models | Students practice constructing representations and models to explain chemical phenomena, including ionic and metallic solids. These models serve as the basis for making claims and predictions about substance behavior. *VSEPR theory does not apply to ionic and metallic solids. |
| Apply VSEPR Theory | Students use VSEPR (Valence Shell Electron Pair Repulsion) theory to draw Lewis structures of molecules, predicting their three-dimensional geometry and polarity. This involves understanding how electron pairs affect molecular shape. |
| Connect Chemical Theories Across Scales | Students can explain connections between atomic-level phenomena and macroscopic properties. For example, they understand how electronegativity and ionization energy relate to bond types and overall substance characteristics. |
| Evaluate Models | Students work with chemical concepts like Coulomb’s law, formal charge, and resonance to assess the accuracy of models in representing particulate-level structures and macroscopic observations. |
| Predict Molecular Properties | Students practice predicting and describing molecular shapes, bond angles, and polarities based on Lewis structures. They also calculate and connect formal charges to the predicted structure of a molecule. |
Note the common mistakes made by students highlighted by CED. They include errors related to valence electrons, octet rule violations, and confusion between molecular geometry and bond angles.
In summary, the AP Chemistry standards require students to have a strong grasp of molecular structure, bonding theories, and the ability to use these concepts to make predictions and support claims about various chemical properties and phenomena. Students are expected to practice these skills extensively to succeed in the course and on the AP Exam.

How to address common mistakes
To help students address common mistakes related to valence electrons, octet rule violations, and confusion between molecular geometry and bond angles, educators can employ various teaching strategies and resources. Here are a few suggestions for teaching “VSEPR for AP chem”:
| Teaching Strategies | Description |
|---|---|
| Clear Visualization | Enhance comprehension through interactive simulations like PhET tools or molecular modeling software. Alternatively, you can opt for tangible materials such as marshmallows with toothpicks or soft foam balls with wires to facilitate learning. |
| Practice with Lewis Structures | Encourage students to practice drawing Lewis structures for various molecules and ions. Emphasize the importance of counting valence electrons correctly and distributing them to satisfy the octet rule. Provide plenty of examples and progressively complex molecules for practice. |
| Resonance Structures | Teach students how to identify and draw resonance structures. Emphasize that resonance structures are different representations of the same molecule, and they contribute to the overall molecular structure. |
| Molecular Geometry vs. Bond Angles | Clarify the distinction between molecular geometry (the spatial arrangement of atoms, including lone pairs) and bond angles (the angles between bonded atoms). Use real-life examples and hands-on activities to reinforce this concept. |
| VSEPR Theory | Introduce VSEPR (Valence Shell Electron Pair Repulsion) theory as a tool to predict molecular geometry and bond angles. Provide practice problems that involve both electron domains and bond angles. |
| Group Work and Peer Teaching | Encourage students to work in groups or pairs to solve problems collaboratively. Peer teaching can be highly effective in reinforcing understanding. |
By implementing some of these strategies and emphasizing the importance of practice, clarification, and a deep understanding of fundamental concepts, educators can help students overcome common challenges in understanding valence electrons, the octet rule, and the relationship between molecular geometry and bond angles.
How Viziscience can help
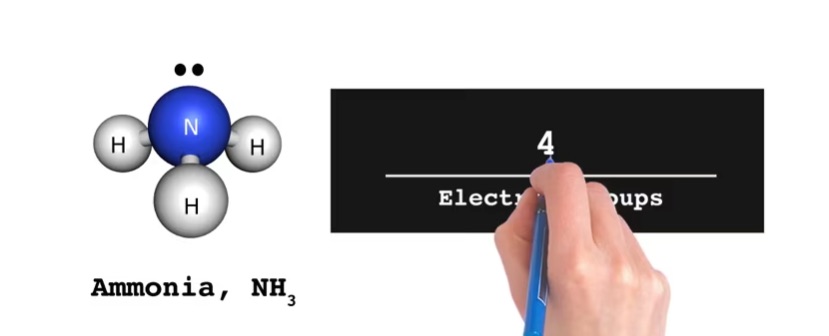
Viziscience offers a comprehensive VSEPR for AP Chem. This unit is ideal for students who are new to chemistry or are looking to quickly progress to the level required for AP Chemistry. This unit is a valuable resource for both teachers and students. It provides a structured and efficient pathway to learning chemistry, complete with high-quality visual aids, educational videos, clear explanatory text, illustrative diagrams, and concept check quizzes.
For teachers, this resource can be a time-saving solution, eliminating the need to piece together materials and lessons. Instead, they can rely on a well-structured and organized unit that covers the fundamentals of chemistry and smoothly transitions students to the AP Chemistry level.
For students, Viziscience’s unit offers an engaging and supportive learning experience. The visual aids and videos make complex concepts more accessible, and the concept check quizzes ensure that students can assess their understanding as they progress.
In summary, Viziscience’s comprehensive unit is a valuable tool that simplifies the process of learning chemistry for both educators and students, making the journey from basics to AP Chemistry level efficient and effective


Your blog is a true hidden gem on the internet. Your thoughtful analysis and engaging writing style set you apart from the crowd. Keep up the excellent work!
Thanks I have recently been looking for info about this subject for a while and yours is the greatest I have discovered so far However what in regards to the bottom line Are you certain in regards to the supply