Reaction mechanism
Chemical reactions often take place in multiple steps and it’s not always obvious from looking at the chemical equation. For example, the decomposition of ozone:
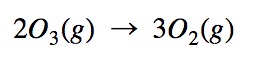
From the equation, it appears that O₃ decomposes to O₂ in one step. In reality, it’s more likely to take place in the following sequence:
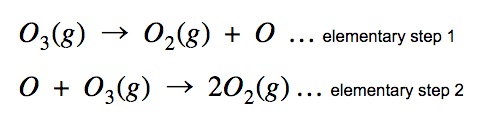
The above shows the decomposition taking place with two steps, we call each step an elementary step. An elementary step is an individual step in the reaction mechanism that cannot be further broken down into smaller steps. Reactions that involve more than one elementary step are called complex reactions.
Intermediates
In the example above, the oxygen atom “O” produced in the first step of the mechanism is consumed in the second step. The entity is known as a “reaction intermediate” and it does not appear in the final chemical reaction.
Rate determining step
How fast can a reaction reach equilibrium or completion? The rate of the reaction is dictated by the slowest elementary step of the reaction. This step is called the “rate-determining step“. No matter how fast the speed of the other elementary steps are, the reaction cannot go faster than the slowest step.
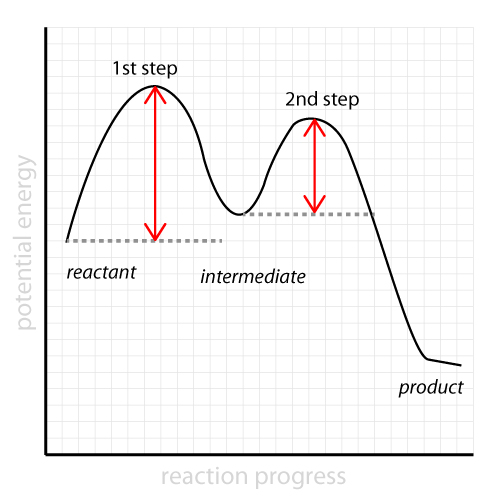
The diagram above is called a reaction coordinate diagram or energy profile diagram. It shows how the energy of the system changes during a chemical reaction.
The rate-determining step is the step with the largest activation energy. In the diagram above, the 1st elementary step is the rate-determining step since it requires more energy for it to proceed forward.
Molecularity
What happens at the molecular level? Molecularity of a reaction tells you how many number of molecules or ions are coming together in a single step reaction to form products.
Reaction mechanism
Suppose you have a reaction given by the equation:
NO₂(g) + CO(g) → CO₂(g) + NO₃(g)
In an experiment (carried out above 225°C), the rate law determined for the reaction was:
rate = k•[NO₂][CO]
In an experiment (carried out below 225°C), the rate law obtained for the reaction was:
rate = k•[NO₂]²
Above 225°C, the reaction is first order with respect to NO₂ and CO. Below 225°C the reaction is second order with respect to NO₂. Suggest a mechanism that is consistent with the above rate laws.
Proposing a mechanism
Sum of individual steps
The sum of the individual steps must sum up to the overall equation. The intermediates do not appear as a final product.
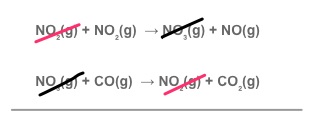
NO₂(g) + CO(g) → CO₂(g) + NO₂(g)

