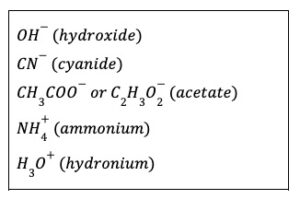
Examples of polyatomic ions
Generally, polyatomic ions have suffixes that end in “ite” or “ate” if they contain oxygen atoms.
| chloride | hypochlorite | chlorite | chlorate | perchlorate |
Note: Chloride shown above is not a polyatomic ion, it's a monoatomic ion consisting of only one atom.
Examples of other polyatomic ions:
Oxyanions
Oxyanions are ions that contain oxygen atoms.
- For example, chlorine can combine with oxygen in 4 ways to form 4 different oxyanions.
- You will notice that the charge in a family of oxyanions remains the same.
- This group of oxyanions has a charge of 1-.
| prefix | HYPO | PER | |||
|---|---|---|---|---|---|
| suffix | ite | ite | ate | ate | |
| anion name | chloride | hypochlorite | chlorite | chlorate | perchlorate |
| #oxygen atoms | 0 | 1 | 3 | 3 | 4 |
| formula | Cl⁻ | ClO⁻ | ClO₂⁻ | ClO₃⁻ | ClO₄⁻ |
| structure |  |  | 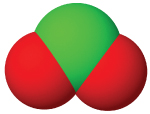 | 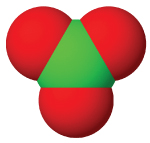 | 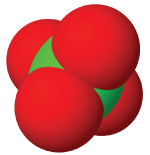 |
Look at the names of each polyatomic ions above.
- Hypochlorite – has the suffix “Hypo”, has the least number of oxygen atoms.
- Perchlorate – has the suffix “Per”, has the most number of oxygen atoms.
Periodic table
The small group of non-metals in the periodic table below commonly form bonds with oxygen, and they are also able to combine with oxygen in a number of ways to form different oxyanions.
Remember, that in a family of oxyanions, the charge remains the same. So, for example, the boron family of oxyanions will have a charge of 3-. Carbon will have 2-, and nitrogen will have 2-. You can find these charges in the periodic table below.
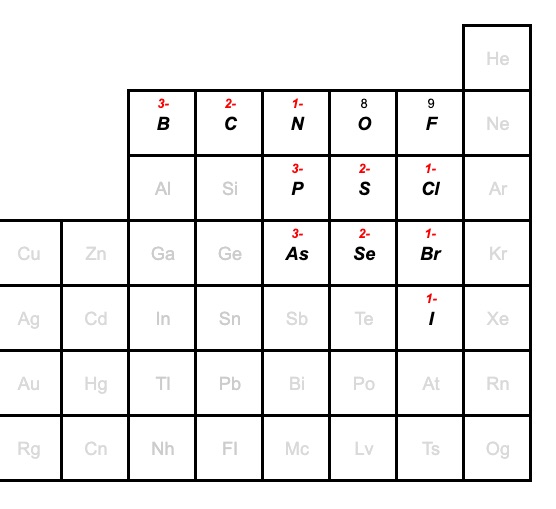
As far as the naming goes, the group of elements (B,C,N,O,F, Br and I) will have naming pattern shown above for the chlorine family.
The nitrogen family
| prefix | HYPO | PER | |||
|---|---|---|---|---|---|
| suffix | ite | ite | ate | ate | |
| anion name | nitride | hyponitrite | nitrite | nitrate | pernitrate |
| #oxygen atoms | 0 | 1 | 2 | 3 | 4 |
| formula | N³⁻ | NO⁻ | NO₂⁻ | NO₃⁻ | NO₄⁻ |
The phosphorus family
The four elements inside the group (P,S,As,Se), their names follow the same pattern but they have an extra oxygen atom.
| prefix | HYPO | PER | |||
|---|---|---|---|---|---|
| suffix | ite | ite | ate | ate | |
| anion name | phosphide | hypophosphite | phosphite | phosphate | perphosphate |
| #oxygen atoms | 0 | 2 | 3 | 4 | 5 |
| formula | P³⁻ | PO₂³⁻ | PO₃³⁻ | PO₄³⁻ | PO₅³⁻ |
Concept check

Concept check

