AP CHEMISTRY TEACHERS – WE HAVE RESOURCES FOR YOUR ENTIRE CLASS TO USE…
Identify if a compound is covalent or ionic
- You will need to know the periodic table
- You must be able to identify the non-metals, metals, and transition elements in the periodic table
- You must be able to categorize a compound as ionic or covalent formed from elements based on their location within the periodic table
- If the substance is an ionic compound, follow the rules for ionic compounds
- If the substance is a covalent compound, follow the rules for covalent compounds
You can download the following flowchart to serve as a guide for naming compounds.
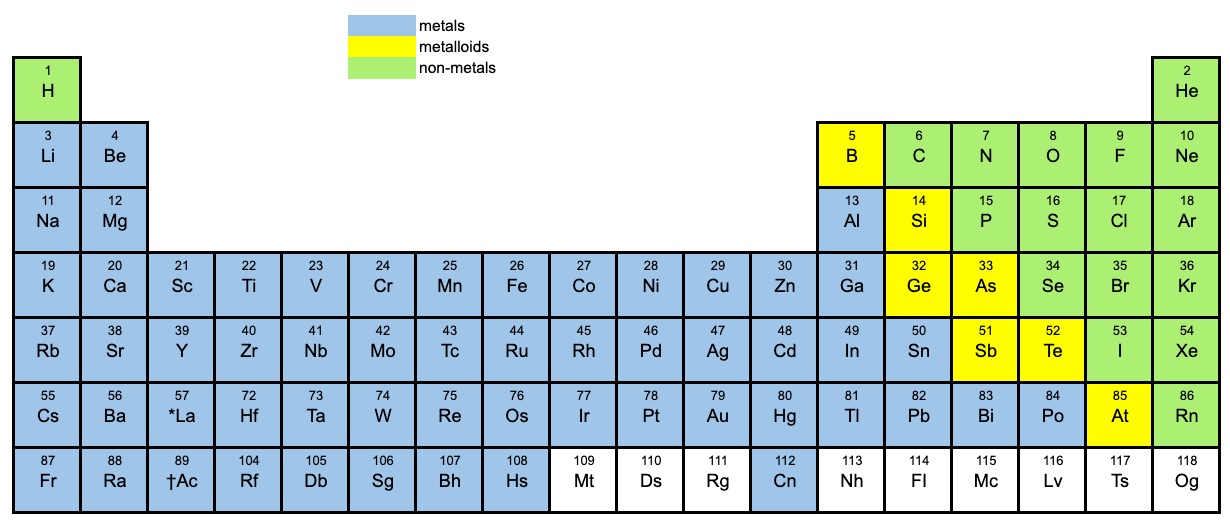
The periodic table
It’s important that you can identify if a compound is a covalent compound or an ionic compound by looking at the periodic table. The periodic table below shows the metals, non-metals and metalloids. Use it to determine if a compound is ionic or covalent.
Compounds
Covalent compounds are generally formed between non-metals.
Eg. Water (H2O), carbon monoxide (CO), hydrochloric acid (HCl)

Ionic compounds are generally formed between a metal and a non-metal
Eg. Sodium chloride (NaCl), potassium fluoride (KF), magnesium oxide (MgO)

Metalloids (semi-metals)
Metalloids: Boron, silicon, germanium, arsenic, antimony, and tellurium.
Metalloids generally form covalent bonds with non-metals. But sometimes, they form ionic compounds with other elements.

SiO₂, silicon dioxide, is a covalent compound.
As₂O₃, arsenic(III) oxide, is an ionic compound.
Next page…
You are ready to go to (2. covalent compounds)
Click on the title below to go to the next page…


cool