How do we determine the number of neutrons in a given atom?
First, let’s define some terms that you’ll need to be familiar with. The atomic number of an atom is the number of protons in the nucleus of that atom. The mass number (also called the nucleon number) of an atom is the total number of protons and neutrons in the nucleus of that atom. Finally, the relative atomic mass (symbol Aᵣ) of an element is the weighted average of the masses of the atoms of every isotope of that element. The unit of this quantity is the atomic mass unit (a.m.u), which is defined as 1/12 of the mass of an atom of carbon-12, that is the isotope of carbon with 6 protons and 6 neutrons.
For the relative atomic mass, we have to take a weighted average because some isotopes are more abundant than others – that means there are more of some isotopes that exist in nature, so the average mass of atoms of that element will be closer to the mass of the isotopes that are more common.
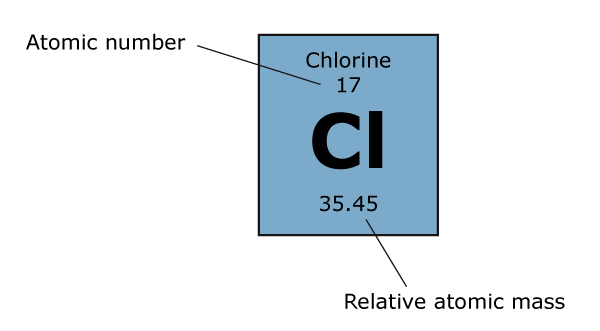
That’s a lot of information – let’s look at chlorine as an example. Chlorine has two isotopes that exist in significant quantity,Cl-35 (75%) and Cl-37 (25%). Remember that the numbers above the symbol are the mass numbers, so Cl-35 has a total of 35 protons and neutrons, and Cl-37 has a total of 37 protons and neutrons. Since we know the atomic number of Cl is 17, we can work out that Cl-35 has 18 neutrons, and Cl-37 has 20 neutrons. Remember – the atomic number of an element is constant for all isotopes, since it is the number of protons that actually defines which element an atom is. To calculate the relative atomic mass of chlorine we have to take the weighted average, so the calculation looks like this: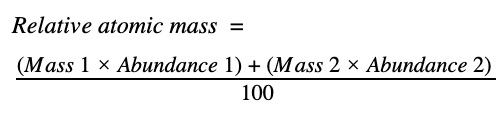
For chlorine, taking the two isotopes Cl-35 and Cl-37, we get: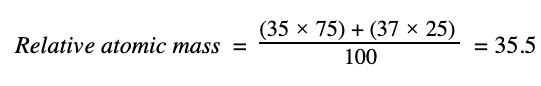
The real answer, 35.45, seen in the periodic table, comes from all the other minor isotopes we didn’t consider, but 35.5 is a good enough answer because all these other isotopes aren’t really relevant. You’ll notice that 35.45 rounds to 35.5 to three significant figures anyway.
Determining the number of neutrons
So what about neutrons? We saw in the chlorine example that for a specific isotope of an element, if we know the mass number of that isotope, we can work out the number of neutrons in an atom of that isotope by taking the mass number (total number of protons and neutrons) and subtracting the atomic number (number of protons). This leaves us with the number of neutrons in that isotope.
But how do scientists work out which isotopes of an element exist in significant proportions? The key is mass spectrometry. Mass spectrometry is a way of measuring which isotopes are present in a given sample of an element. If you take a mass spectrum of chlorine, for example, you get a graph that that looks like this:
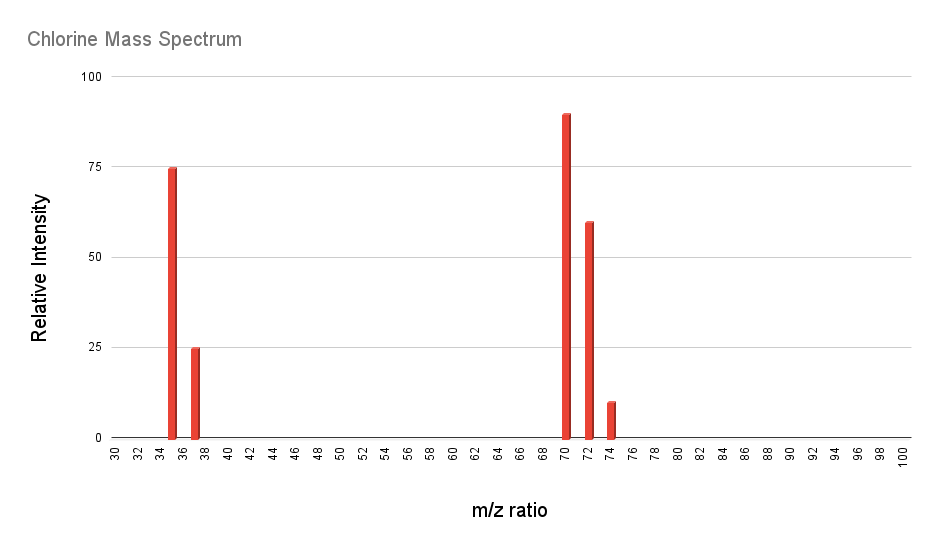
The y axis corresponds to the relative abundance of each signal, the x axis is the mass to charge ratio, m/z. For this spectrum the charges are all just +1, so this axis just corresponds to the mass of the isotope that gives each signal. We know that chlorine exists as a diatomic gas, Cl2 – that’s why we see signals way up around 70. The most interesting part is the two signals at 35 and 37. These correspond to atoms of chlorine, specifically the isotopes Cl-35 and Cl-37. The abundances are approximately in the ratio 3:1, and this is how a scientist would work out which isotopes exist and in which relative quantities they are present. The above spectrum is simplified a little bit, as there would be very small quantities of other isotopes as well, but we don’t need to worry about these.
Author’s name: Ronan Haskurti
Title: BA (Hons) Natural Sciences
University: University of Cambridge , UK
| How to Find the Number of Neutrons in an Oxygen Atom | |
|---|---|
| Yes ✅ (Mass Number Given) | No ❌ (Mass Number Not Given) |
| Step 1: Look at the given mass number (e.g., Oxygen-16). | Step 1: Find the atomic mass of oxygen from the periodic table. |
| Step 2: Find the atomic number (Z) for oxygen from the periodic table (Z = 8). | Step 2: Round the atomic mass (15.999) to the nearest whole number (16) to estimate the mass number. |
Step 3: Use the formula: Neutrons = Mass Number (A) - Atomic Number (Z) |
Step 3: Use the formula: Neutrons ≈ Rounded Mass Number - Atomic Number (Z) |
| Step 4: Since you are given the exact mass number, you now have the exact number of neutrons. | Step 4: Since this is an approximation, the neutron count may slightly vary in real isotopes. |
|
Example: Oxygen-16 – Given Mass Number (A) = 16 – Atomic Number Z = 8 – Neutrons = 16 – 8 = 8 ✅ (Exact) |
Example: Oxygen – **Atomic Mass = 15.999** (from periodic table) – Rounded Mass Number ≈ 16 – Atomic Number Z = 8 – Neutrons ≈ 16 – 8 = 8 ✅ (Estimate) |

