Enthalpy change
For a reaction
 |
Bonds
Know that
- Breaking bonds always requires energy (never releases energy)
- Forming bonds always releases energy (never requires energy)
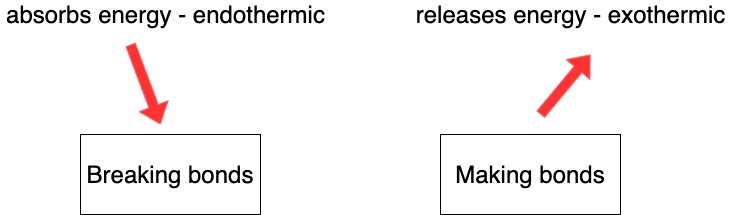 |
Exothermic and endothermic process
- Breaking bonds is always an endothermic process (whether breaking single, double or triple bonds).
- Forming bonds is an exothermic process (whether making single, double or triple bonds).
However, the overall reaction may be exothermic or endothermic depending if the amount of energy used for breaking bonds is greater or less than the amount of energy released when bonds are formed.
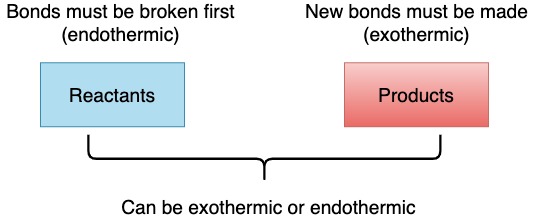 |
Example – formation of water
Calculate the energy required to perform this reaction:
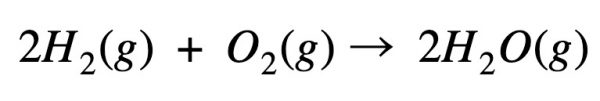
| Bond | Average Bond Enthalpy
(kJ mol-1) |
| H–H | 436 |
| O=O | 495 |
| O–H | 463 |
Calculations
You can use a table with 2 columns to differentiate the bonds that are broken and bonds are made.
| Bonds broken (reactants)
kJ mol-1 |
Bonds made (products)
kJ mol-1 |
|---|---|
| 2(H–H) = 2(436) | 4(O–H) = 4(463) |
| 1(O=O) = 495 | |
| Sum ΔH = 1367 kJ mol-1 | Sum ΔH = 1852 kJ mol-1 |
In this method, you do not need to worry about the signs of the energy change.
ΔH of reaction
ΔHrxn = Sum(ΔH bonds broken) – Sum(ΔH bonds formed)
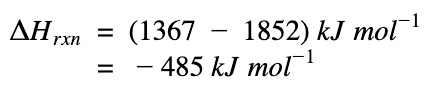 |
The negative sign means heat is given out. The reaction, formation of water, is an exothermic process.
If the reaction gives off heat, then the surrounding will gain the heat lost by the reaction and becomes warmer.
Units
The unit for ΔHrxn per mole can be expressed in either format:
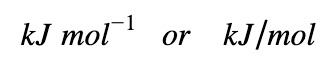 |
Common mistakes
Here’s a list of some of the common mistakes to avoid when doing bond energy calculations.
![]() Student forgets to make sure the equation is balanced before attempting the question
Student forgets to make sure the equation is balanced before attempting the question
![]() Student counts wrongly the number of bonds.
Student counts wrongly the number of bonds.
- Example, in 2 moles of H2O there are 4 (O–H) bonds and not 2 because each mole has 2 (O–H) bonds.
![]() Student not using the formula in the right order so the sign is wrong
Student not using the formula in the right order so the sign is wrong
- Bonds broken first before bonds made
- Always use Sum(ΔH bonds broken) – Sum(ΔH bonds made)
![]()
- If the calculations shows a negative value, it means heat is released. Student calculates the value correctly but does not put in the negative sign so the answer will be regarded as wrong.

