Background: How ionic compounds are formed
Ionic compounds are formed from the electrostatic attraction of oppositely charged ions.
The ions are arranged in such a way that they form a giant crystal lattice of alternating positive and negative ions.
An example of an ionic compound is sodium chloride, NaCl, also commonly known as table salt.
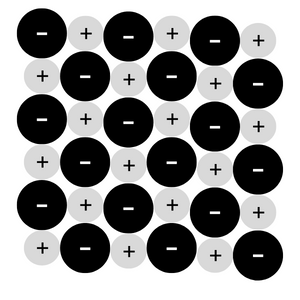
An ionic compound lattice
- Metals tend to lose electrons to non-metals.
- Non-metals tend to gain electrons from metals.
- The atom that loses electrons becomes a positively charged ion – called a cation.
- The atom that gains electrons becomes a negatively charged ion – called an anion
- When the cations and anions come close together, they experience an attraction due to their opposite charges.
- The bond that is formed between two oppositely charged ions is called an ionic bond.
Balance charges
It’s important to learn how to balance the charges in ionic compounds
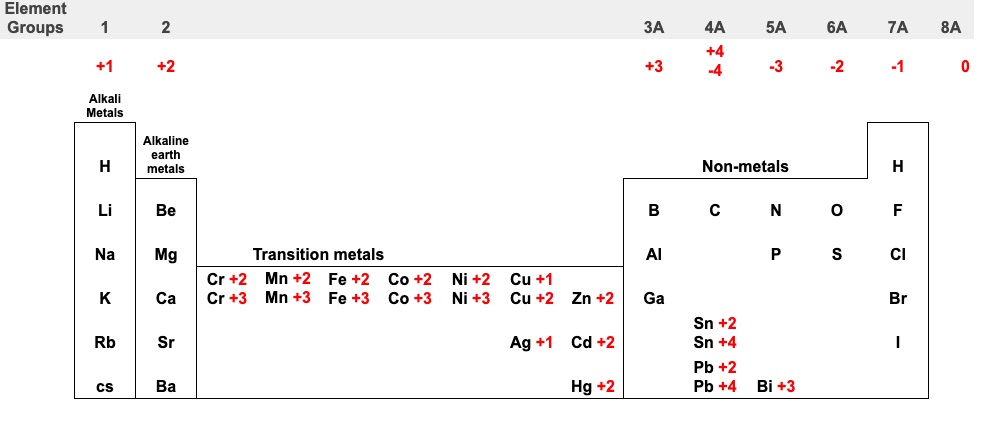
- Use the periodic table to determine the charge in an element
- The overall charge in an ionic compound must be neutral
- All the negative charges must cancel out all the positive charges in the compound
Ionic charges
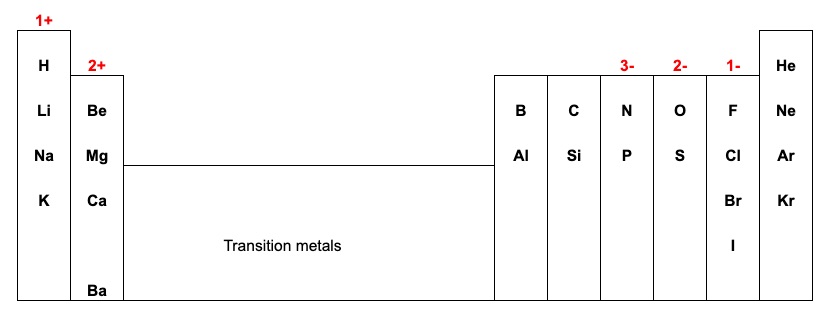
Note: All elements in the first column tend to lose 1 valence electrons and form ions with 1+ charge All elements in the 2nd column tend to lose 2 valence electrons and form ions with 2+ charge
Example #1 – sodium and chlorine
Look at the periodic table to obtain the ionic charges of sodium and chlorine. Balance the charges using the table below:
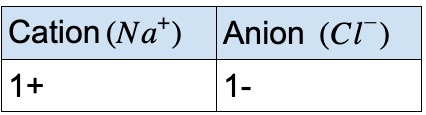
Formula: NaCl
Example #2 – calcium and chlorine
Look at the periodic table to find the ionic charges of calcium and chlorine:
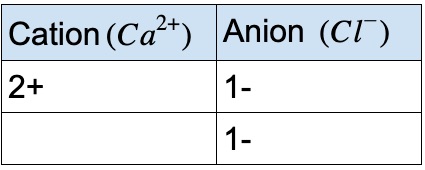
The net overall charge must be 0, so we need 2 Cl ions to balance the charge of 1 Ca ion. The ionic formula is CaCl₂ Subscripts in the chemical formula indicate the number of atoms.
Rules for ionic compounds
- First, determine the formula by balancing the charges
- The cation is always named before the anion
- The second element ends in “IDE”
- If transition elements are involved, you must use Roman numerals for elements with more than one oxidation state.
Rules for transition metals
- • In naming the transition metal oxides, add a Roman numeral in parenthesis straight after the name of the transition metal ion.
- • Do not put a space in between.
- • The Roman numeral must have the same value as the charge of the ion. Then add the name of the anion – “IDE” at the end.
Ionic charges for transition metals
Transition metals’ charges vary but the charges are always positive.
Iron (Fe) has possible charges of 2+, 3+, 4+, 5+ and 6+, but it commonly forms compounds with 2+ and 3+ charges. You must balance the charges the same way as before.
Compound containing Iron and chlorine
If the iron 2+ ion combines with chloride, what is the formula of the compound?
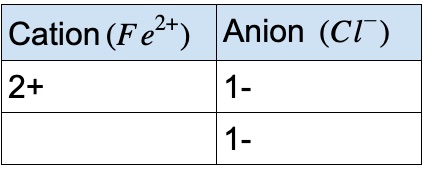
- • Look at the periodic table
- • Chlorine has 1- charge as an ion
- • To balance the charge on the cation, 2 chlorine ions are needed
- • Therefore, the ionic formula is FeCl₂
- • Fe is a transition metal, we must use a roman numeral to indicate the charge
- • The compound name is: Iron(II) Chloride
If the iron 3+ ion combines with chloride, what is the formula of the compound?
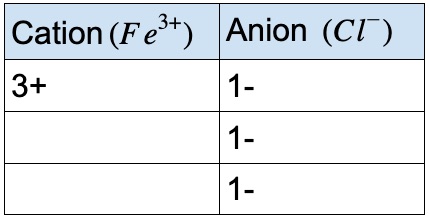
We need three Cl– anions to balance the charge of one Fe3+cation.
- • The ionic formula: FeCl₃
- • To name the compound, we must use roman numerals
- • The compound name is: Iron(III) Chloride
Compound containing lead and oxygen
Lead has 2+ charge. Oxygen has 2- charge. What is the ionic formula?

- • The ionic formula is PbO
- • The name of the compound is Lead(II) oxide since lead has a charge of 2+
Compound containing silver and chlorine
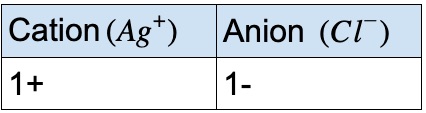
- First, identify the type of compound it is.
- It falls in the category of ionic compound because it has a metal (Ag) bonded to a non-metal (Cl)
- Refer to the periodic table above, Ag forms 1+ charge and Cl forms 1- charge.
- So, the compound is silver(I) chloride, but it is commonly known as silver chloride.
Transition metals that do not require Roman numerals
Roman numerals are only used for transition metals that have more than one ion. For example, Fe forms Fe2+ or Fe3+ ions in compounds. The transition metals below do not need a Roman numeral in the names of their compounds because they only form one ion. Cd, Zn, Al
Find Roman numeral
To find the Roman numeral for the element, first refer to the periodic table.
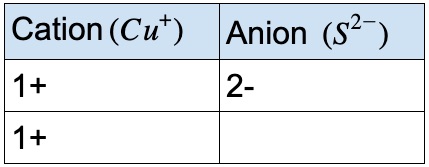
- • For example, Cu, can have a charge of 1+ or 2+
- • Sulfur will always have a charge of 2-
- • The table below shows that 2 Cu+ will balance the charge of 1 S2-.
- • Therefore, the Roman numeral (I) must be used to show the charge of the copper ion.