Bond Enthalpy
Introduction
In order to understand why breaking bonds requires energy (endothermic) and making bonds releases energy (exothermic), one must first understand the nature of a chemical bond and why it occurs in the first place.
Remember that bonds are due to the exchange or sharing of electrons between atoms. Forming bonds is a very favorable interaction because the driving force is to reach stability by having a stable outer shell configuration.
Favorable and unfavorable
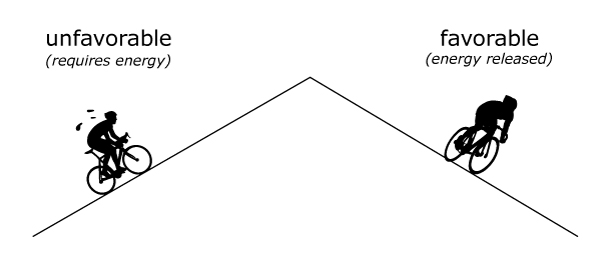
Think of bond breaking and formation like going up and down a mountain. Going up the mountain is unfavorable – it requires a lot of energy input. Going down the mountain is favorable – it releases energy.
 |
Bonds breaking and formation
So, when we want to break a bond, we need to put in energy because it is unfavorable – atoms are less stable when separated. When forming bonds, atoms try to achieve stability, and this favorable process actually releases energy.
 |
Endothermic or exothermic
The energy input or release due to the breakage or formation of a bond is involved in determining whether a reaction is endothermic or exothermic. Since bonds come in all different strengths, they require differing amounts of energy to be broken, and release different amounts of energy when they form.
This difference in bond strengths means that the energy required to break reactant bonds will differ from the energy released upon product formation. As a result, some reactions are endothermic (require energy input) and some are exothermic (release energy).
Bond energies
Next, we go into the details of how bond energies are used to calculate reaction enthalpies.
https://viziscience.com/bond-energies/calculating-bond-energies/